How Do I Know Which Oxidation State to Use
The ion is more properly called the sulfate VI ion. In water N H X 4 X 2 M o O X 4 is dissociated in 2 N H X 4 X M o O X 4 X 2.

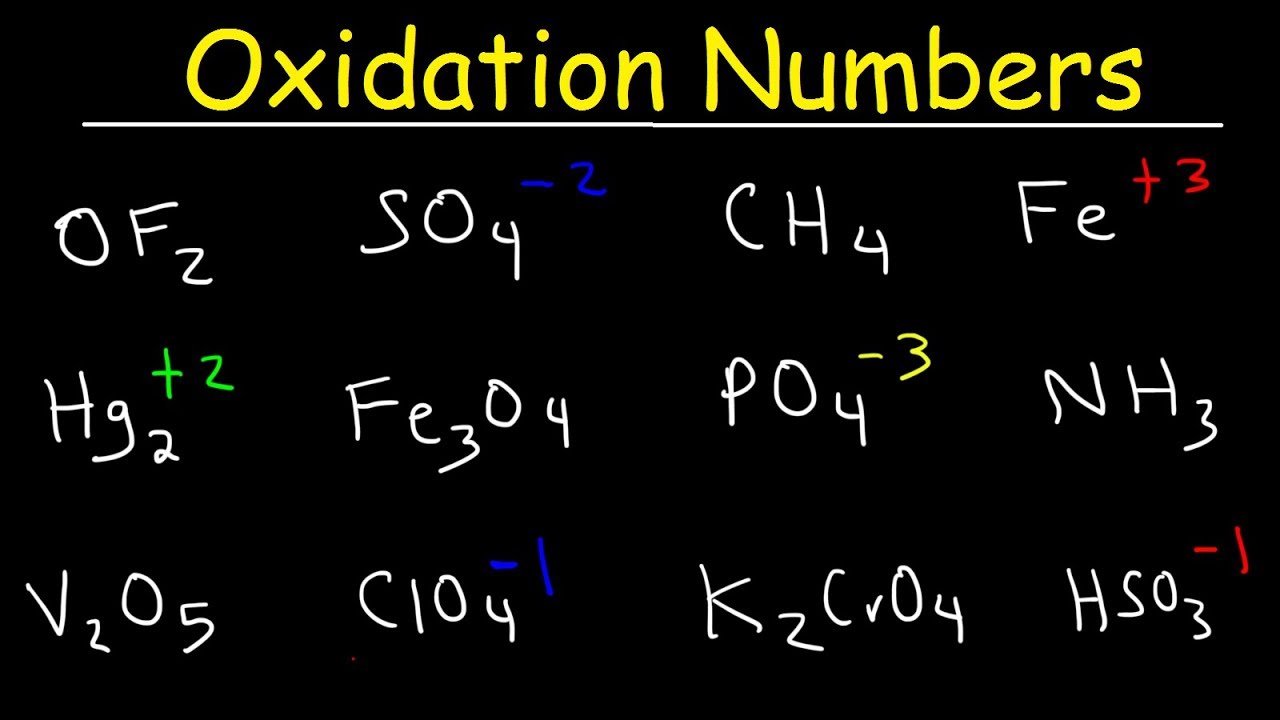
How To Find Oxidation Number Oxidation State Chemtalk
We use what rules we can to determine the oxidation numbers.
. In HCl the H is H. The sum of the oxidation states of all the atoms or ions in a neutral compound is zero. Therefore oxidation number of oxygen in SO2Cl2 is -22-4.
Metals in all compounds have a positive oxidation state. To determine the oxidation number of an atom in a molecule you must start from the molecules electron dot diagram. The modern names reflect the oxidation states of the sulfur in the two compounds.
Rules to determine oxidation states The oxidation state of an uncombined element is zero. The sulfite ion is SO 32-. They allow chemists to do things such as balance redoxreductionoxidation equations.
Using oxidation states In naming compounds You will have come across names like iron II sulphate and iron III chloride. In some cases the average oxidation state of an element is a fraction such as 8 3 for iron in magnetite Fe 3O 4 see below. Oxidation states are typically represented by integers which may be positive zero or negative.
1 -1 0 since its not an ion it adds up to zero. Height weight and oxidation state Like how everyone can be described by their height and weight every element has. The atoms in He and N 2 for example have oxidation numbers of 0.
In ions the algebraic sum of the oxidation states of the constituent atoms must be equal to the charge on the ion. The oxidation state of. Any free element has an oxidation number equal to zero.
We know Oxygen generally shows a oxidation number of -2. If you want a more direct interpretation in terms of a physical property. In the ammonium N H X 4 X cation Nitrogen is at oxidation number x and H at 1.
The oxidation state loosely corresponds to electron density around an atom relative to what it would be like if that atom was neutrally charged and isolated. The oxidation number of H is 1 but it is -1 in when combined with less electronegative elements. Xe Cl 2 S 8 and large structures of carbon or silicon each have an oxidation state of zero.
Reduction occurs when the oxidation number of an atom becomes smaller. So -4 -2X0Therefore X6. The highest oxidation state of an element is determined using the periodic table by the group in which it is located.
Let the oxidation number of S be X. Rule 8 states the numbers along the bottom must add up to zero. For a given bond X-Y the bond is split to give X and Y where Y is more electronegative than X.
Generally the oxidation state for most common elements can be determined from their group number on the periodic table. The convention is that the cation is written first in a formula followed by the anion. The algebraic sum of oxidation states for all atoms in a neutral molecule must be zero.
Also Cl has an oxidation number of -1. The oxidation numbers are 436 H 2 1 O 2 1. Oxidation occurs when the oxidation number of an atom becomes larger.
The oxidation state of carbon increases from 2 to 4 while the oxidation state of the hydrogen decreases from 1 to 0. Free elements have an oxidation state of zero Free elements are pure elements. In addition to that you must apply the following rules to successfully use the molecules electron dot diagram to.
Group 2A elements alkaline earth. Oxidation and reduction are therefore best defined as follows. The sulfate ion is SO 42-.
Oxidation state of oxygen -2. Oxidation state of sulphur in H 2 SO 5 x 2 2 -1 1 0 x 6 c Oxidation number of P in H 3 PO 4. Here each ion can be analyzed separately to get the unknown oxidation numbers.
Rule 7 states that the oxidation number of Cl is -1. We write the oxidation number of the element above its symbol and the total for 3 Cl atoms below the symbol. In compounds with non-metals hydrogen has an oxidation state of 1 and an oxidation state of -1 with metals.
The oxidation state of the sulfur is 6 work it out. Looking at the OH group alone oxygen gets -1 oxidation state H gets 1 oxidation state. The oxidation number of a free element is always 0.
The highest known oxidation state is reported to be 9 in the tetroxoiridium IX cation IrO 4. The oxidation numbers are 435 O 2 F 2 1 e In Hydrogen peroxide H 2 O 2 the OH bond pairs are assigned to the more electronegative Os but the OO bond is purely covalent and the electron pair is divided equally. Now the overall charge is 0.
This chemistry video tutorial provides a basic introduction on how to calculate oxidation numbers. In the anion M o O X 4 X 2 the same calculation yields 6 for. Group 1A elements alkalai metals always have an oxidation of 1.
The oxidation number of a mono-atomic ion is equal to the charge of the ion. This gives Cr-1 Cl3 mmmmmmll-3mm. ELEMENTS Oxidation numbers are bookkeeping numbers.
An oxidation number can be assigned to a given element or compound by following the following rules. You will know that it is 2 because you know that metals form positive ions and the oxidation state will simply be the charge on the ion. H 2 F 2 2 HF The overall reaction may be written as two half-reactions.
The hydrogen atom H exhibits an oxidation state of 1. Therefore oxidation number of Cl2 in SO2Cl2 is -12-2. For example in NaH the H is H-.
This applies regardless of the structure of the element. Oxidation state of peroxo linkage -1. The oxidation number of a monatomic ion equals the charge of the ion.
It discusses how to find the oxidation states of elements. For monoatomic ions the oxidation number always has the same value as the net charge corresponding to the ion. The average oxidation state of S is 5 0 0 54 104 25 b Oxidation number of S in H 2 SO 5 Here the oxidation state of OH -1.
There are a set a rules that we use to determine oxidation number. This gives each O seven electrons a gain of 1 over the neutral atom. Answer 1 of 3.
So that x 4 1 1 so that x 3. H 2 2 H 2 e the oxidation reaction F 2 2 e 2 F the reduction reaction There is no net change in charge in a redox reaction so the excess electrons in the oxidation reaction must equal the number of electrons consumed by the reduction reaction. The oxidation number of a free element is always 0.
Same old same old. But each OH group has a spare space on each oxygen to grab another electron so each oxygen in each OH group can get to. How to find oxidation state.
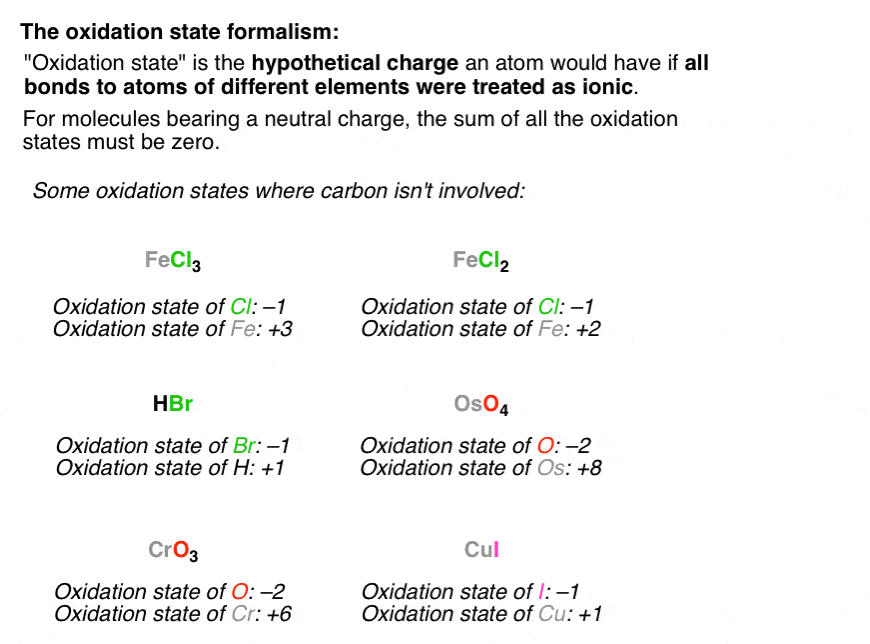
Calculating The Oxidation State Of A Carbon Master Organic Chemistry

Calculating Oxidation Number Youtube

How To Calculate Oxidation Numbers Basic Introduction Youtube
No comments for "How Do I Know Which Oxidation State to Use"
Post a Comment